The need for precision health products and services is increasing as people are paying more and more attention to their own health as a result of better living standards, and aging populations and rising medical costs are driving the demands as well.
Thanks to the rapid development of DNA sequencing technology, we now have a better understanding of the human DNA code and can offer customized precise health assessment and management solutions for individuals with different genotypes, gene expressions, environments, lifestyles, and molecular basis of disease.
Using Precision Medicine to Promote Overall Wellness
Nowadays, not only do patients benefit from medical-related technology, but healthy individuals can also use precision health technology to safeguard their health; and precise testing, prevention, and diagnosis are all essential to the growth of precision healthcare.
Personalized healthcare is generated by introducing the technologies during different stages (before, during, and after) of the disease. For example, early detection of disease risks, observed changes in health status, and physiological monitoring data are used to create personalized health management schemes to prevent illness; and precise diagnostics can be employed as a disease screening and identification support tool to help with follow-up treatment decisions as well as relapse tracking and monitoring.
In addition, the rise of smart city concepts has accelerated the development of remote medical care and Internet of Medical Things (IoMT) around the world, which not only supports citizens’ health and welfare but also stimulates national economies. Along with the continuous evolution of personalized services and home health business models, it is foreseeable that health evolution products such as genetic testing and wearable monitoring devices will become more common and eventually become part of our daily lives.
Developing More Precise Multigene Panel Testing
Quark Biosciences was established in 2012 at the ITRI Incubation Center in response to the clinical needs of precision medicine. The company combined biotechnology with optoelectronic semiconductor technology to develop a molecular diagnosis system that can quickly and accurately detect multiple genetic markers simultaneously.
This system uses highly sensitive multigene panel tests to provide personalized health evolution solutions that overcome the shortcomings of traditional “one size fits all” treatments. The personalized medical treatments take the genetic characteristic differences of each individual into account and thus reduces expensive testing procedure costs and overall medical expenses.
Moving away from the complicated traditional next-generation sequencing, Quark Biosciences developed the miRNA (microRNA) technology which can screen hundreds of biomarkers at once. In addition to providing features such as simple operations and multi-dimensional analysis, the system also looks at the gene characteristics of the individual when providing a customized health evolution solution.
The multigenic quantitative testing platform is already used in precision medicine screening for lung cancer, breast cancer, diabetes, thyroid nodules, immunotherapy response prediction, and reproductive medicine. Results can be promptly acquired with a tiny sample to support diagnosis and medication guidelines.
Integrating Technologies, Building Platforms, and Developing Products
Quark Biosciences offer unique technology that can detect changes in miRNA gene regulators. It is known that changes in gene expressions are closely related to cancer, cardiovascular diseases, and diabetes; therefore, by using miRNA as a molecular detection marker, we can obtain information about the individual's health faster and more accurately.
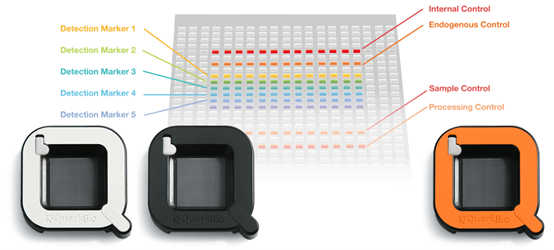
Quark Biosciences developed a specific chip (photo 1) with semiconductor and microchannel designs exclusively for the detection system. This chip can amplify signals and detect a single gene among different miRNA biomarker probes in thousands of nanometer-sized reaction wells. The wells do not interfere with each other, which improves accuracy and sensitivity. This allows the system to complete information analysis of 100 gene expressions within 2 hours for each batch.
Photo 1. PanelChip ® Multigenetic Testing and Analysis Chip (Image source: Quark Biosciences’ official website)
To further enhance detection capabilities and expand application scope, Quark Biosciences conducted big data analyses on gene expressions related to disease diagnosis, treatment, and physiology and established a miRNA biomarker database (miDatabase) platform to develop new miRNA detection targets.
Quark Biosciences has been developing innovative molecular detection technology for more than 10 years and a number of products have successively entered the market since 2019. Up to now, the company has amassed about 50 international patents and is the only company in Taiwan developing miRNA clinical testing products and systems.
Since 2017, Quark Biosciences has been collaborating with Taoyuan Min Sheng General Hospital, ACT Genomics, and Amwise Diagnostics to develop miRNA cancer screening products to further promote innovative detection technologies and platforms as well as commercialize these technologies. The company launched the first miRNA Endometrial Receptivity Analysis (ERA) testing tool in the world in 2021 with Genetics Generation Advancement Corp (previously: Genesis Genetics Asia), breaking into the lucrative fertility testing market. This product, which can improve the success rate of therapeutic insemination, is designed and developed by Quark Biosciences and commercialized and marketed by Genetics Generation Advancement Corp.
Combining Cross-domain Technologies to Advance Precision Health Care
ITRI has a wide array of professional cross-domain technologies that can support the development of precision health care, including photoelectric sensor technology, information algorithm analysis, multi-detection solutions, and comprehensive multiomics evaluation. Integration of these technologies can improve hard/software system efficiency and computing accuracy as well as deliver timely health solutions for different individuals.
ITRI has long been involved in the R&D of related technologies with the goal of building domestically-produced gene detection products and machines. ITRI successfully launched startups like Quark Biosciences and Personal Genomics by utilizing cutting-edge technology available at the time, such as optoelectronic semiconductors, biomarker exploration, and microarray gene chips. The startups went on to develop innovative miRNA multigene panel testing products and high-efficiency monomer sequencing machines that use optoelectronic semiconductor technology.
Recently, ITRI is developing relevant hardware and software technologies to accelerate the actualization of precision medicine. In the area of biochips, ITRI built a rapid fixed-point detection system that features newly designed microchannels integrated with magnetic droplet control functions. This system can automatically complete biomarker detection within 30 minutes. ITRI also leveraged microelectromechanical processes and packaging and testing technologies to trial-produce testing chips with CMOS circuits and sensor components, consequently reducing the product’s time to market. In data analysis, ITRI uses bioinformatics systems, machine learning, and data mining to determine the relationship between diseases and the optimal treatment pathway. With additional supporting technology, it is expected to greatly reduce the development time of new drugs.
Taiwan has outstanding medical care quality and health insurance data resources. Looking forward, collaborations between ITRI and the biomedical optoelectronics industry are expected to galvanize upgrades in local medical technology industries and advance the health evolution of mankind.